View 43++ Quality System Manual Pdf Solve Your Solution
Table of Contents
If you’re searching for quality system manual pdf images information linked to the quality system manual pdf interest, you have come to the right site. Our site frequently gives you hints for seeing the maximum quality video and picture content, please kindly surf and find more informative video articles and graphics that fit your interests.
Quality System Manual Pdf. One of the key purpose of implementing a quality management system is to act as a preventive tool using. Ad PDF signer to quickly complete and sign any PDF document online. The quality management system documentation includes. X Consistently provide products and services that meet customer and applicable regulatory requirements.
 Iso 9001 2015 Requirements Summary Of Each Section From the9000store.com
Iso 9001 2015 Requirements Summary Of Each Section From the9000store.com
Wilson rockwell hardness tester manual pdf Yamaha outboard service manual pdf download free Yale pallet jack manual pdf Toyota corolla repair manual pdf
X Consistently provide products and services that meet customer and applicable regulatory requirements. It is designed to be modified to reflect your organisation. All copies of this Quality Management Systems Manual QMSM must be kept under strict control to prevent the system from becoming unreliable. Documents including records determinedby the organization to be necessary to ensure the effective planning operation and control of its processes and 5. Ad PDF signer to quickly complete and sign any PDF document online. 422 Quality Manual The Quality Assurance Department is responsible for the management of the quality manual.
The review is held at a minimum of once per year and includes assessing opportunities for improvement and the need for changes to the quality management system.
PDF PPT Documents in a Few Clicks Anytime from Anywhere. All copies of this Quality Management Systems Manual QMSM must be kept under strict control to prevent the system from becoming unreliable. 04 MANAGEMENT OF QUALITY MANUAL 041 Purpose The Quality Manual is the prime reference document used for implementing the QMS. This Quality Manual specifies the general requirements for Millennium competence towards a management system for quality administrative and technical operations. This Quality Manual is established maintained and identifies the manner in which Lamons addresses each specific requirement of ISO 900115 API 9Q1th Edition20 and other technical specifications. 422 Quality Manual The Quality Assurance Department is responsible for the management of the quality manual.

Credit: id.pinterest.com
Audit results customer feedback status of corrective. 421-01 Rev 12 2202018 Page 2 of 13 QMS Manual. 04 MANAGEMENT OF QUALITY MANUAL 041 Purpose The Quality Manual is the prime reference document used for implementing the QMS. This documentation includes the Quality Manual. Ad PDF signer to quickly complete and sign any PDF document online.

Credit: pinterest.com
BTD Quality System Policy Manual - 2015 1 Page 7 of 19 442. The purpose of the Quality Manual is to document the quality system and policies and to inform ALSPIs customers of the controls implemented to assure product quality. The Quality Manual Policies and Procedures or documented processes work instructions forms and records. It is designed to be modified to reflect your organisation. It should be used as a template only.

Credit: the9000store.com
ISO 90002015 Quality management systems Fundamentals and vocabulary ISO 9001 Fifth Edition 2015-09-15. Quality Management System QMS Manual This manual has been documented around Mango Limiteds certified Management System. And ensuring these documents objectives plans and standards are current. Ad PDF signer to quickly complete and sign any PDF document online. ISO 90002015 Quality management systems Fundamentals and vocabulary ISO 9001 Fifth Edition 2015-09-15.

Credit: pinterest.com
422 Quality Manual The Quality Assurance Department is responsible for the management of the quality manual. The purpose of the Quality Manual is to document the quality system and policies and to inform ALSPIs customers of the controls implemented to assure product quality. ISO 90002015 Quality management systems Fundamentals and vocabulary ISO 9001 Fifth Edition 2015-09-15. X Consistently provide products and services that meet customer and applicable regulatory requirements. It should be used as a template only.

Credit: pinterest.com
The review is held at a minimum of once per year and includes assessing opportunities for improvement and the need for changes to the quality management system. The official controlled copy of this quality manual is the digitally signed PDF document held within our network. It should be used as a template only. No scan print required. It is designed to be modified to reflect your organisation.

Credit: pinterest.com
The Quality System Manual is a controlled document that is maintained by the Management Representative and distributed. Below is a list of the required proceduresdocumented processes. Quality Management System QMS Manual This manual has been documented around Mango Limiteds certified Management System. It is designed to be modified to reflect your organisation. The Quality Management System Manual is the principal document covering the requirements of ISO 90012015 requirements and incorporates applicable elements of the NSQHS Standards and is supported by facility policies procedures and forms.

Credit: docplayer.net
This Quality Manual is established maintained and identifies the manner in which Lamons addresses each specific requirement of ISO 900115 API 9Q1th Edition20 and other technical specifications. Consideration during the development of this Quality Policy Manual. The Quality Management System Manual is the principal document covering the requirements of ISO 90012015 requirements and incorporates applicable elements of the NSQHS Standards and is supported by facility policies procedures and forms. Quality Management System QMS Manual This manual has been documented around Mango Limiteds certified Management System. It is designed to be modified to reflect your organisation.

Credit: pinterest.com
Developed by CLSI and are organized as the 12 Quality System Essentials. Documented policies and objectives regarding quality performance This quality management system manual Documented procedures refer to Appendix A3 Documents required or the effective planning operation and control our processes Quality records and data The level and type of management. A quality system manual 3. No scan print required. - Management reviews the quality system to ensure its continuing suitability adequacy and effectiveness.

Credit: the9000store.com
Consideration during the development of this Quality Policy Manual. ISO 90002015 Quality management systems Fundamentals and vocabulary ISO 9001 Fifth Edition 2015-09-15. Top Management ensures the organization. This quality manual is the property of Your Company. X Consistently provide products and services that meet customer and applicable regulatory requirements.

Credit: examples.com
1 Scope This Quality Manual specifies requirements for a quality management system where Millennium. Quality Manual Template wwwiso9001helpcouk ISO 90012015 Quality Management System Document Ref. Documents including records determinedby the organization to be necessary to ensure the effective planning operation and control of its processes and 5. One of the key purpose of implementing a quality management system is to act as a preventive tool using. Ad PDF signer to quickly complete and sign any PDF document online.

Credit: id.pinterest.com
The Quality Management System Manual is the principal document covering the requirements of ISO 90012015 requirements and incorporates applicable elements of the NSQHS Standards and is supported by facility policies procedures and forms. And ensuring these documents objectives plans and standards are current. It must not be reproduced in whole or in part or otherwise disclosed without prior written consent. 04 MANAGEMENT OF QUALITY MANUAL 041 Purpose The Quality Manual is the prime reference document used for implementing the QMS. The Quality Management System Manual is the principal document covering the requirements of ISO 90012015 requirements and incorporates applicable elements of the NSQHS Standards and is supported by facility policies procedures and forms.

Credit: docplayer.net
- Management reviews the quality system to ensure its continuing suitability adequacy and effectiveness. A diagram representing these 12 essentials is shown below. Quality Manual Template wwwiso9001helpcouk ISO 90012015 Quality Management System Document Ref. 04 MANAGEMENT OF QUALITY MANUAL 041 Purpose The Quality Manual is the prime reference document used for implementing the QMS. 80 Quality System Documentation Structure The quality system employed within Kickhaefer Manufacturing Company consists of several tiers of documentation such as.

Credit: pinterest.com
All copies of this Quality Management Systems Manual QMSM must be kept under strict control to prevent the system from becoming unreliable. And ensuring these documents objectives plans and standards are current. The following controls will ensure that the system remains current and valid. All copies of the manual will be clearly numbered and the Holder recorded. The quality management system documentation includes.
Credit: medium.com
1 Scope This Quality Manual specifies requirements for a quality management system where Millennium. One of the key purpose of implementing a quality management system is to act as a preventive tool using. Quality Management System QMS Manual This manual has been documented around Mango Limiteds certified Management System. Page 17 of 51 6 Management System Planning 61 Addressing Risks Opportunities In order for our organization to have a successful quality management system we. It should be used as a template only.

Credit: pinterest.com
This Quality Manual specifies the general requirements for Millennium competence towards a management system for quality administrative and technical operations. The Quality System Manual is a controlled document that is maintained by the Management Representative and distributed. Below is a list of the required proceduresdocumented processes. Documents including records determinedby the organization to be necessary to ensure the effective planning operation and control of its processes and 5. To define the scope of our QMS including details of and justification for the requirement of standard that the organization determines is not applicable to the scope of quality.

Credit: id.pinterest.com
422 Quality Manual The Quality Assurance Department is responsible for the management of the quality manual. This Quality Manual is established maintained and identifies the manner in which Lamons addresses each specific requirement of ISO 900115 API 9Q1th Edition20 and other technical specifications. The Quality System Manual will be reviewed and approved by the President and the ISOIATF Management Representative of the facility. B Retains documented information to have confidence that the processes are being carried out as planned. The review is held at a minimum of once per year and includes assessing opportunities for improvement and the need for changes to the quality management system.

Credit: pinterest.com
And ensuring these documents objectives plans and standards are current. The Quality System Manual is a controlled document that is maintained by the Management Representative and distributed. The Quality Management System Manual is the principal document covering the requirements of ISO 90012015 requirements and incorporates applicable elements of the NSQHS Standards and is supported by facility policies procedures and forms. Documented procedures and records required by standards listed in section 20 4. All copies of this Quality Management Systems Manual QMSM must be kept under strict control to prevent the system from becoming unreliable.
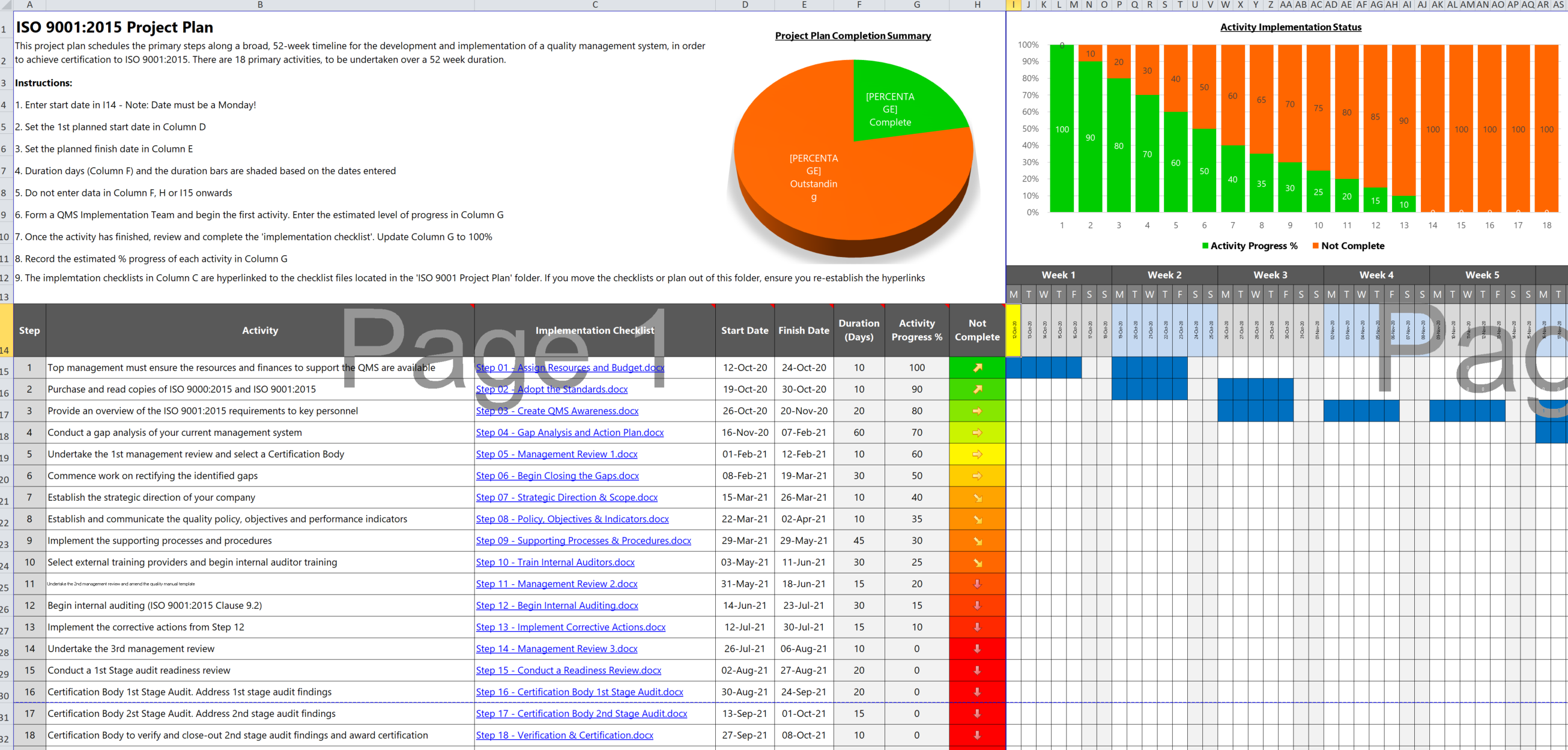
Credit: iso-9001-checklist.co.uk
No scan print required. The Quality System Manual is a controlled document that is maintained by the Management Representative and distributed. And ensuring these documents objectives plans and standards are current. All copies of this Quality Management Systems Manual QMSM must be kept under strict control to prevent the system from becoming unreliable. Documented policies and objectives regarding quality performance This quality management system manual Documented procedures refer to Appendix A3 Documents required or the effective planning operation and control our processes Quality records and data The level and type of management.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title quality system manual pdf by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.
